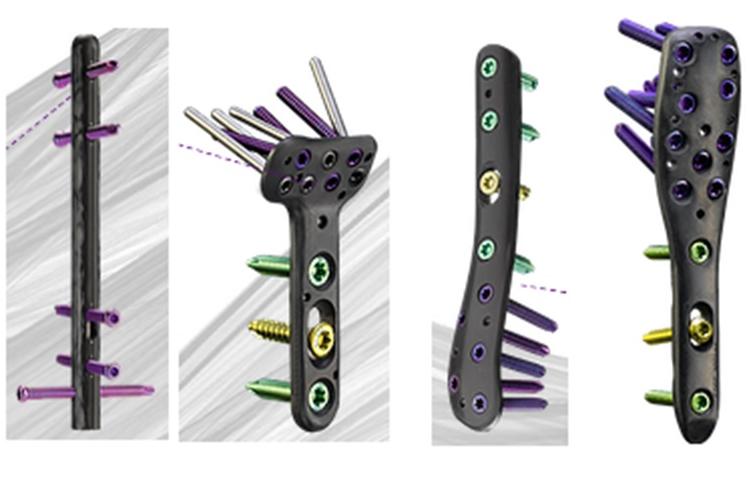
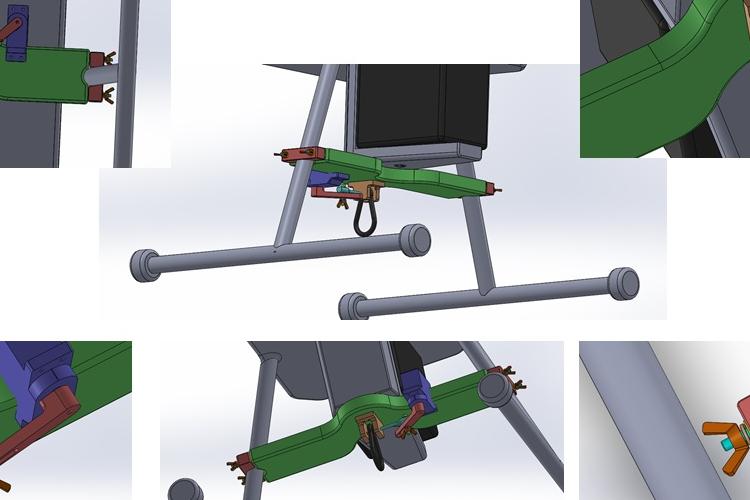
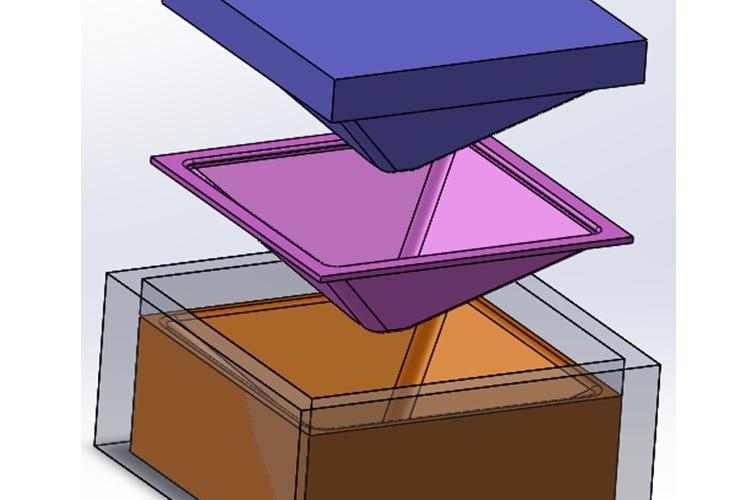
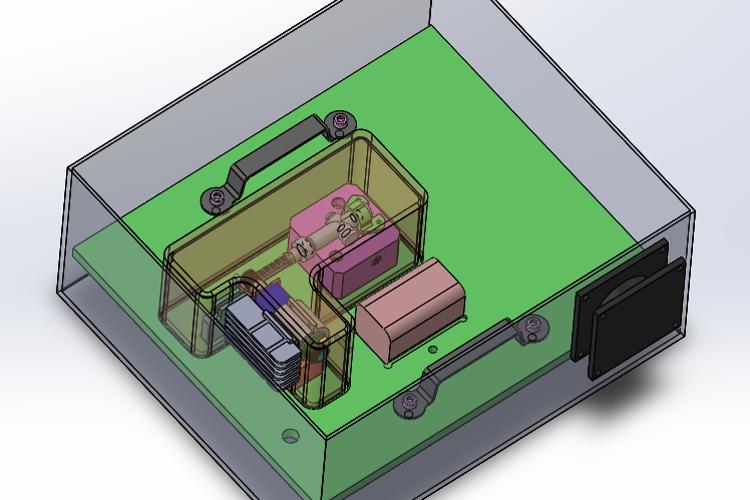
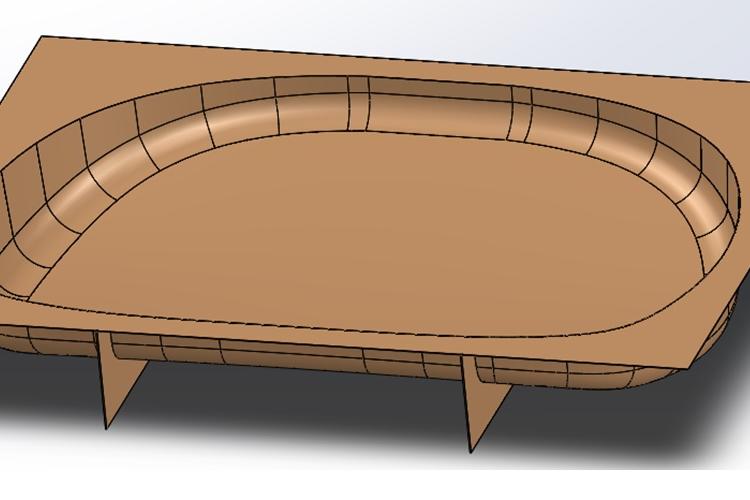
Biomedical Engineering
•Established & initiated the products technology including all process development & integration of the multidisciplinary technologies for the products production.
•Established the R&D division, including in house development and outsourced services work methodologies & more.
•Established & initiated the production lines in house & at subcontractors, in Israel, Europe & USA.
•Responsible for all product research, development and engineering aspects including: design, testing, documentation, regulatory submissions, and product improvements.
•Integration of the development processes with all company departments: Marketing, clinical, QA/RA & operation.
•Preparation of design history files and submissions to notified bodies and FDA Compliance with medical standards: ISO 13485, FDA and others.
•Chief scientist: submission preparation and main part of the approval process.








 Post Project
Post Project


 Continue with Facebook
Continue with Facebook
 Continue with LinkedIn
Continue with LinkedIn




 Invite
Invite